Have a look at these two glaciers, one has fresh snow over the glacier and the other is a dry glacier in summer with accumulated deposits of dust and Black Carbon from air pollution. Which one do you think is more vulnerable to melting? Does a bright white surface reflect more or less light than a darkened surface?

Fresh clean snow on the Silvretta glacier, Switzerland (Zoë Fleming)

Dirty ice on the Fox Glacier, New Zealand (Sylvia Knight)
Particulate Matter is solid particles that are so small that they float in the atmosphere. They are formed from incomplete combustion of wood and fossil fuels. When they are smaller than 2.5 microns (2.5 x 10-9 m, an eight the width of a human hair), this PM2.5 can enter into our lungs and be carried into the blood system and cause damage to the brain and the cardiovascular system.
When Particulate Matter (or Black Carbon, which is more or less soot or pure Carbon) settles on glaciers and snow it darkens the colour of the snow and hence changes the how much of the Sun’s light the snow reflects. In this experiment we will check to see whether dirty or clean ice melts faster.
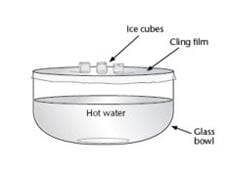
Materials
Chemicals | Apparatus |
3 ice cubes per group | 3 bowls for placing ice cubes |
Soot or Activated Carbon or burn a splint and gather the blackened combusted material | Spotlight |
Soil or sand (as light coloured as possible) | Measuring scale |
| Spoon or forceps to move the ice cube between the bowl and the measuring scale |
Method
- Take 3 ice cubes out of the freezer and place one in each bowl.
- Scatter soot over the ice cube in one bowl, covering it completely. Scatter the next ice cube with the soil. The last bowl will contain the control ice cube.
- Weigh each ice cube (using a spoon or forceps to place it on the scale).
- Shine the light bulb over the 3 bowls, trying to equally light/heat them all.
- After 5 minutes, remove each ice cube one at a time to weigh them.
- After 10 minutes, remove each ice cube one at a time to weigh them.
- If you have time to wait for the first ice cube to completely melt, note the time and note down how much was left of the other ice cubes (weigh them).
Results and Questions
- Which ice cubes melted faster?
- Does covering them with brown or black melt them faster?
- Thinking about a sunny day on snow, how do your eyes react to the sunlight? Does it seem like there is more light around or less than on a sunny day walking on bare soil? What about a sunny day on a boat? Do you think there is more or less light reflected back to your eyes than on land? The proportion of the Sun’s light which is reflected by a surface is called its albedo – a high albedo means a large proportion of the light is reflected and, therefore, only a small proportion is absorbed.
- What about the difference between wearing white or black clothes on a sunny day- which one absorbs the sun rays and makes you feel warmer? Is that a small or large albedo?
Application for the world’s glaciers:
Glaciers around the world are more exposed to particulate matter now than they ever were before the industrial revolution. Covering them with a dark material changes the albedo. The darker the surface, the more of the Sun’s light is absorbed by the glacier, warming it and melting it.
Particulates are tiny solid or liquid particles that are present in the atmosphere. They are sometimes termed aerosols as they float in the air. Black carbon (soot) is a particulate released from incomplete combustion. It absorbs the Sun’s light, which actually helps to cool the Earth. However, when it lands on ice, the absorption of radiation speeds up the ice´s melting as the light is absorbed by the dark colour and heats up the ice.
Social and political perspectives:
Knowing that air pollution that reaches glaciers is increasing their melting faster than what would happen from air temperature changes alone, what do you think we can do in terms of laws or behaviour change?
How can we reduce soot and Black Carbon reaching glaciers? Emission control of cars? Banning domestic wood-burning? Have you heard of smokeless coal that can be used in stoves in smoke-free zones? And pellet stoves, are there fewer emissions from these?
Note: You could carry out your own experiment if you are lucky enough to get snow. Prepare two neat snow blocks or two snow-balls of similar size and cover one with gravel or sand and leave the other clean. Watch which one melts first.